650-575-5935
3D Fluorescent Microscopy
July 2021
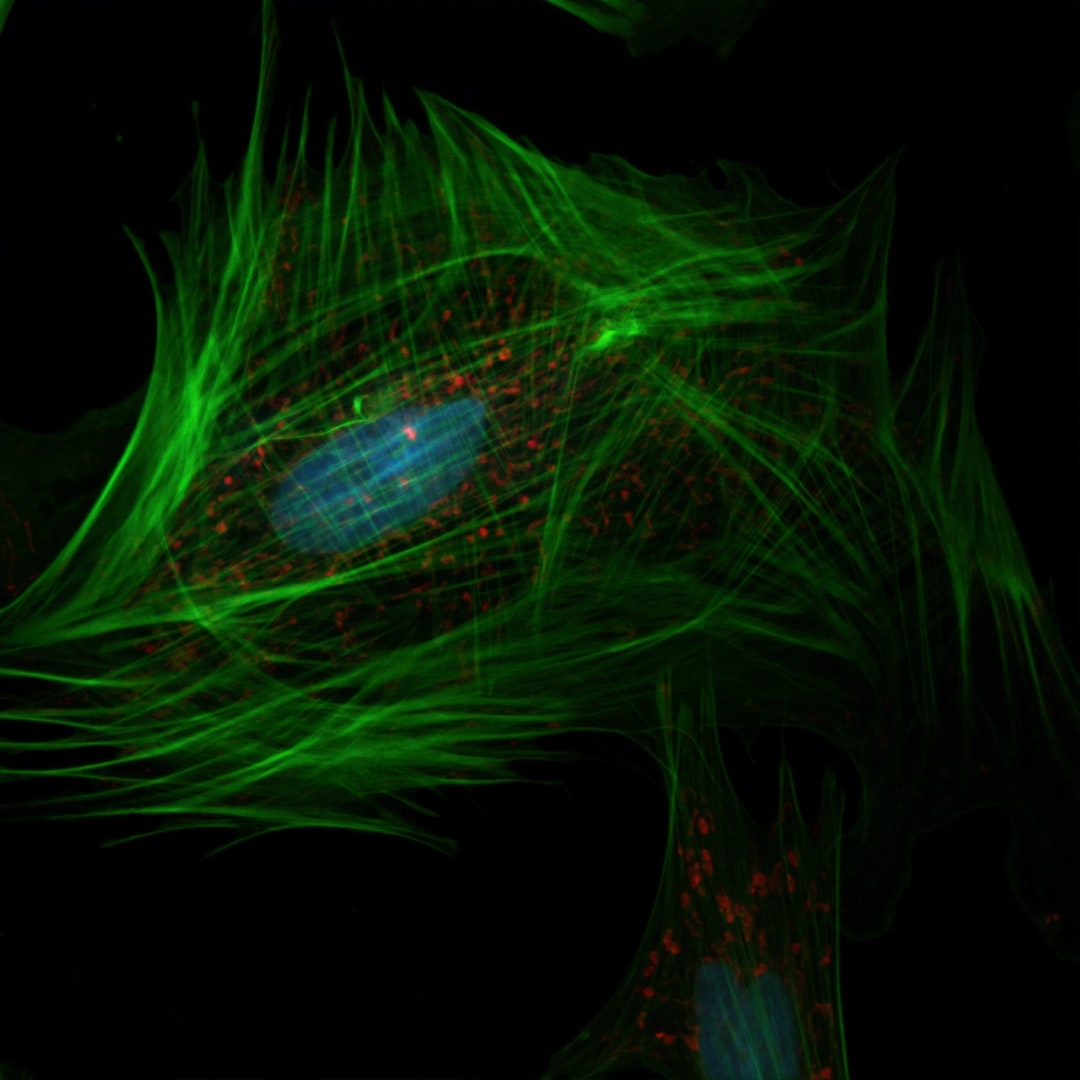
Fluorescence microscopy remains one of the most common and most informative methods of analyzing biological samples. It gives important spatial information, essential to our understanding of biological processes and underlying structures or interactions. It is routinely used in life science laboratories by trained scientists and technicians in a multitude of applications, in both commercial and academic settings.
Microscopy techniques primarily provide two-dimensional spatial information about the specimen. The advances in microscopy hardware (i.e., confocal microscopy, light-sheet fluorescence microscopy) have opened the door to more informative three-dimensional (3D) imaging, and with it, improve our understanding of the true nature of cellular and tissue structures and processes. 3D imaging is now finding use in a wide range of applications in life sciences research, from drug development to disease modeling. It is used with a growing variety of samples, ranging in size from subcellular to organ-level (cleared animal organs, tissues, or 3D cell culture models such as organoids and spheroids).
The great potential of 3D imaging notwithstanding, the adoption of 3D microscopy in a wider range of applications does face some technical challenges. One of them is the inability to efficiently visualize the internal regions of the progressively bigger and thicker samples. As light travels through the biological samples, it is diffracted by the media. The longer the path light needs the travel, the larger the degradation of the signal and image quality. Improvements in microscopy hardware and computational analysis are working on addressing this issue. The other recourse is to improve the quality of the sample. One way to address this is by sample “clearing”. This is done by matching refractive indices in the media and removing biological components, such as fats, that significantly contribute to light diffraction. The other cause of low signal in the interior of large samples has to do with how biomarkers are detected. The samples are typically processed using the same sample staining methodologies developed for thin sections. This practice tends to produce partial or poor staining results, primarily due to the slow penetration of the visualization dyes or antibodies into the sample. This problem gets progressively worse in thicker and/or denser samples. In order to mitigate this issue, users routinely either extend staining incubation times, from hours to days or even weeks or significantly increase the concentration of the reagents used in the assay. These workarounds are both impractical and expensive. An effective solution to this problem will enable faster sample processing, cost reduction, better image quality, and more accurate data, and thereby help champion the much-needed adoption of 3D microscopy.